WHO is not currently working. A large part of that is due to the vaccine production issues that nearly all Pharma companies are dealing with. Another part is that the funding of COVAX has been too slow. The Trump Administration refused to take part initially and the US only came on board this year following the change in administration.
However, the ramping up of vaccine production is taking place. 100s of millions of vaccines are being produced and made available through the COVAX scheme for countries that would otherwise have no chance whatsoever of either developing or procuring vaccines in those numbers. It needs to improve substantially (and it will) but it’s much better than it would be without it.
No, the UK is not refusing to export vaccines or vaccine components. Pharma companies are responsible for exports (unless there is a government imposed ban, such as in the US or export measures such as the EU have set up) and all Pharma companies based in the UK are fulfilling their orders.
It is not the same as there is no UK ban on exporting vaccines or vaccine components. The EU have already banned at least one shipment to Australia and refused direct pleas to allow the lawful export of much needed vaccines to Papua New Guinea.
The EU is making a choice. The EU is not producing these vaccines or exporting them, companies based in the EU are. Lives will be (and are being) sacrificed by the EU banning the export of certain vaccines lawfully ordered by other countries. It is now choosing to say that European lives are more valuable and that vaccines lawfully ordered from Pharma companies based within the EU should be sequestered and diverted, unlawfully, to the EU.
The UK is not doing this, at all. On the contrary, the only reason that AZ is able to produce in the volumes it currently is, is a consequence of the prior agreement with the UK. The UK has also ensured that the Oxford vaccine has been licensed around the world for production and distribution, at cost. It has ensured that the IP is exported, for no profit, so that manufacturing can take place on a mass scale and in as many places as possible.
Again you are suggesting that speed implies recklessness or insufficient scrutiny. This is simply not the case. The same mechanism would be available to EU member states - I would be surprised if the EU would legislate for something that would be so potentially reckless. It also misunderstands how the MHRA was able to approve AZ and Pfizer quicker than the EMA. It worked alongside these companies, was effectively updated in real time, it meant that it was not looking at a whole load of data in one go when it came to final approval but was effectively reviewing the data it had already had oversight of for months. That makes the process no less safe but a whole lot faster. The EMA had the same data to work with when it too approved AZ for use.
The EMA has approved the Janssen vaccine for use ahead of the MHRA. Does that mean it’s been reckless and that Janssen has not been subject to sufficient scrutiny? Of course not.
It’s worth bearing in mind that the UK has a lot of expertise in regulatory approval. The EMA was, after all, based in the UK up until Brexit.
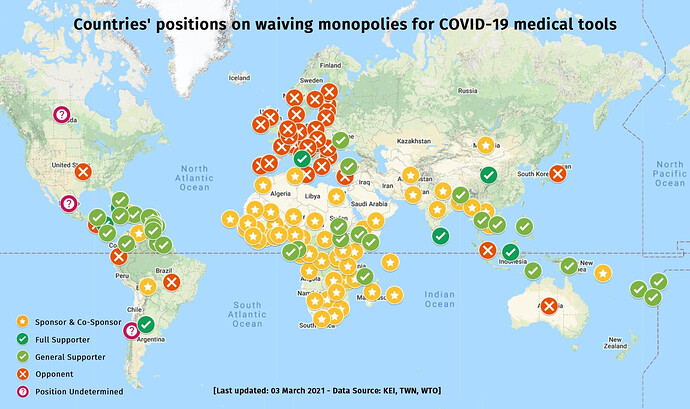
 Those fictional post-apocalyptic storylines of absolute selfishness are coming to pass.
Those fictional post-apocalyptic storylines of absolute selfishness are coming to pass.


 .
.