Fixed it for you ![]()
You beat me to it! 
It would just fit the zeitgeist perfectly to have the whole dispute turn on the definition of ‘Europe’.
Yeah 
I was thinking the same thing - first port of call will be the interpretations section!
It’s still irrelavent to your initial claims. Which were incorrect!
As far as I know the Sanofi mRNA project was looking at having most of the production in the USA which is why there were concerns that it would preveledge the USA when/if produced.
As has been pointed out the GSK/Sanofi project is very different!
My ‘claim’ was that Sanofi had production capacity for their vaccine that fared poorly in Phase 1 and 2 trials, and that capacity was now being repurposed for Pfizer/Biontech production from that vaccine. My question was why that production capacity was not diverted to support production of Pfizer or Moderna earlier.
You then decided that that was a comment on the Sanofi mRNA vaccine in development, and that I was therefore incorrect. My question was always about why the recent announcement by Sanofi was so late, given that neither of their candidates are anywhere near production.
My guess is AstraZeneca may have signed 2 incompatible contracts.
One contract with the UK - UK first.
And then one contract with the EU - including UK sites for the vaccine delivery.
Ramifications for all multinationals and added layers of mistrust between UK/EU.
I noticed the sudden spike in posts in this thread, knew it’s not about something good. 
Does this refer to the Pfizer vaccine - I saw someone yesterday say that the terms of their agreement with the US was that they had to share it’s details with the US army.
What’s this hyper-bowl he’s talking about?
It’s when the Super Bowl winners- the world champions- play a team from another planet.
I think.
The DPA is a two-step process. Companies are notified under the powers of the DPA. That actually happened in December for Pfizer. The next step is ‘rated orders’, i.e. a production order that compels the recipient to prioritize fulfillment of the order for the US government, and also offers legal protection accordingly. Pfizer has not actually been served with a ‘rated order’ (or at least as of Friday, last info I saw), because there isn’t really a need. The Pfizer plant in Kalamazoo is already processing only that one order.
Becton-Dickinson (the syringe manufacturer) has been notified and served with a rated order since inauguration, in order to produce the syringes needed to get 6 doses from the Pfizer vials.
Here is a law firm’s primer on the DPA and related executive order
For interest only at this point (as AZ/EU APA not yet published) this is how reasonable best efforts is defined in the Curevac/EU APA (my emphasis, although the whole paragraph is important),
‘Reasonable best efforts’: a reasonable degree of best effort to accomplish a given task, acknowledging that such things as, without limitation, the complex and highly regulated nature of the Product; the timely availability of
raw materials, inventories and liquid funds; yield of process; the success of necessary clinical trials programs to support safety and immunogenicity data for the Product; the approval of the final Product formulation;
contractor’s commitments to other purchasers of the Product; other reasons relating to the uncertainties of producing a new vaccine for a new disease with an mRNA platform for which vaccines have not yet been registered by regulatory authorities; and any other currently unknown factors which may delay or render impossible, contractor’s successful completion of the particular task, including without limitations, developing a suitable production process as may be required for a new strain of virus, ramping up capacity at contract manufacturing partners, meeting delivery schedules and obtaining the EU marketing authorisation may be beyond the complete control of the contractor, provided, however, that the contractor shall not be required to take any actions inconsistent with past practice, ordinary course of business, prudent and reasonable business behaviour and/or the contractor’s budget plannings at the date hereof.
You said the Sanofi recombination vaccine was a none starter which was a false claim and implied it was an mRNA vaccine which is also false, so false info. You have not rectified that!
The production capacity that Sanofi has offered Pfizer is only for bottling, as stated before there’s enormous bottling capacity in France and will add across Europe. If Pfizer had bottling capacity problems the real question is why didn’t they procure the such earlier? Why wait for Sanofi or other to offer?
As this won’t be up and ready until July it has to be seen as surplus to any Sanofi needs.
AZ should just say the paperwork is too difficult to export due to Brexit ![]()
From 13 January this year
Q1704 Aaron Bell: I see the EU is hoping to make a decision on your vaccine by the 29th as well. Are those doses potentially from the same supplier or are they going to be supplied from other sites elsewhere?
Tom Keith-Roach: No. We have built dedicated supply chains across the world to fulfil our contractual responsibilities. We have a dedicated UK supply chain for drug substance manufacture and for fill and finish. I am delighted to say that the majority of API for the UK come from UK‑based suppliers, and the vast majority of our filling, finishing and packaging also is being done in the UK. It is a dedicated supply chain and our ability to commit to that 100 million doses is not affected by approval status in other supply chains.
I did not use the word recombination, I referred to the Sanofi statement regarding their delay back to Q4-2021, which has allowed them to do the production deal. Pretty damn close to a non-starter for me, though to be fair they have not completely pulled the plug on it yet. Maybe Phase 2b will produce better results.
I made no reference to their mRNA vaccine, you did. I referred to their mRNA production capability. Since Sanofi and Pfizer seem to be in agreement that this will increase production capacity by 100M doses, it seems relevant.
I find it hard to believe it is just a bottling deal - in which case I do agree with you. That seems an utterly bizarre production step to hold back output of an additional 100M.
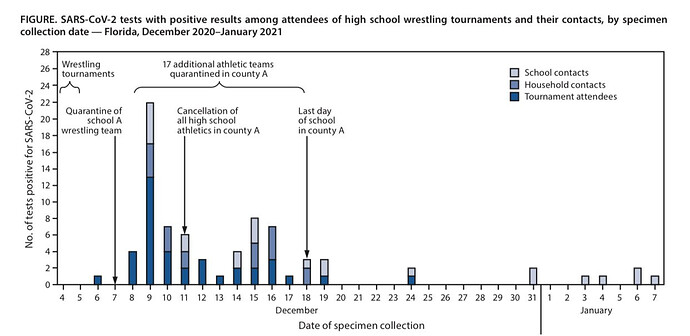
Who ever could have guessed that wrestling would have the potential for spreading the virus?
Absolute madness.